Zofran Cleft Palate
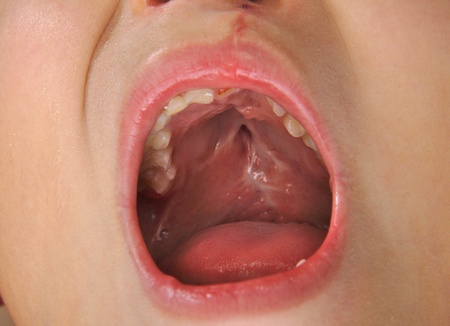
Babies who are exposed to Zofran in the womb during the first trimester of pregnancy may be twice as likely to develop a cleft palate compared to those who haven’t been exposed. A growing number of families are now pursuing Zofran cleft palate lawsuits that accuse GlaxoSmithKline of concealing the medication’s alleged association with major birth defects.
Zofran Birth Defects Investigation
The nationwide law firm of Bernstein Liebhard LLP is now investigating Zofran lawsuits involving cleft palate, cleft lip and other congenital abnormalities. If you believe that Zofran harmed your baby, please contact our Firm by calling (888) 994-5118.
What is a Cleft Palate?
A cleft palate is birth defect that forms in the early weeks of pregnancy. The malformation develops when the tissue that makes up the roof of the mouth fails to join together completely during the sixth and ninth week of fetal development. For some children, both the front and back parts of the palate are open. For other babies, only part of the palate is open.
According to the U.S. Centers for Disease Control, about 2,650 babies are born with a cleft palate every year in the U.S. The cause of cleft palate in newborns is unknown. However, studies indicate that certain risk factors can increase the likelihood that a child will develop a cleft palate or lip, including smoking, diabetes and the use of certain medications.
Children born with a cleft palate may have difficulty with eating and speaking, as well as hearing problems and issues with their teeth. Potential cleft palate complications include:
- Dysphagia (problems swallowing)
- Speech and language development delays
- Frequent ear infections
- Hearing problems
- Nasal-sounding speech
- Missing teeth
- Dental and orthodontic problems
Surgery to correct a cleft palate is generally recommended within the first 18 months after birth, and many children will require additional surgeries as they grow older. Special dental or orthodontic care, as well as speech therapy, might also be required.
Zofran and Cleft Palate
Zofran, an anti-nausea medication marketed by GlaxoSmithKline, has been frequently prescribed to expectant mothers during the first trimester to help alleviate the nausea and vomiting that sometimes occurs with pregnancy. However, Zofran has never been approved for this indication, and its impact on a developing pregnancy has not been well-studied. Nevertheless, one recent analysis indicated that around 1 million pregnant women annually in the U.S. are prescribed Zofran or a generic equivalent for this purpose.
In recent years, a growing body of research has suggested a link between the use of Zofran and the development of major birth defects, including cleft palate:
- 2006: The authors of Placental Transfer of Ondansetron During Early Human Pregnancy concluded that Zofran’s active ingredient breaches the human placental barrier during the early months of pregnancy, the same period of time that a cleft palate will form.
- November 2011: A scientific paper appearing in Birth Defects Research linked Zofran to a 2.4-fold increased risk of cleft palate.
- 2013: Scientists examining records from 900,000 pregnancies included in a Danish health registry concluded that women who took Zofran during the first trimester were 30% more likely to have a baby with a major birth defect.
Pennsylvania Mom Discusses Her Son’s Zofran Cleft Palate Nightmare
A Pennsylvania mom claims that her son’s first few days of life were a nightmare, all because of a cleft palate he allegedly developed due to Zofran. “Struggling, sitting up, wondering and crying every night if your baby is going to make it through the next night,” Ashley Wright recently told Pittsburgh’s WPXI-TV. Though the 10-year-old is doing better today thanks to treatment, he still suffers some lingering effects from his birth defect. Read More
Zofran Birth Defects Litigation
Mounting Zofran birth defects claims have been filed in U.S. courts since February 2012, including a number of cleft palate cases. In October 2015, all federally-filed Zofran cases were consolidated in a multidistrict litigation that is now underway in the U.S. District Court, District of Massachusetts. More than 100 lawsuits were pending in the proceeding as of November 16, 2015. Read More.
Am I Eligible to File a Zofran Lawsuit?
You may be able to file a Zofran lawsuit if you were prescribed this medication in your first trimester and gave birth to a baby with a cleft palate. To obtain a free case review from an attorney at Bernstein Liebhard LLP, please call (888) 994-5118.
- CDC (2014) “Facts About Cleft Lip and Palate” http://www.cdc.gov/ncbddd/birthdefects/cleftlip.html
- AJOG (2014) “Treating morning sickness in the United States—changes in prescribing are needed” http://www.ajog.org/article/S0002-9378%2814%2900853-9/abstract
- Clinical Phamokinetics (2006) “Placental transfer of ondansetron during early human pregnancy” http://www.ncbi.nlm.nih.gov/pubmed/16584287
- Birth Defects Research (2011) “Medications Used to Treat Nausea and Vomiting of Pregnancy and the Risk of Selected Birth Defects” http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3299087/
- TDM Journal Club ( 2013) “Scary Science: Ondansetron Safety in Pregnancy—Two Opposing Results From the Same Danish Registry” http://www.helpher.org/blog/wp-content/uploads/2014/01/2014-Koren-Ondansetron-Safety-TDM.pdf
Get the latest news and litigation updates about this case by following us on Facebook. Click the "Like" button below.
Follow Us


