Zofran Cleft Lip
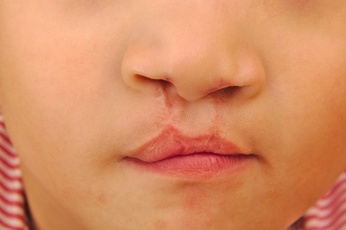 Expectant mothers who are prescribed Zofran off-label to control nausea and vomiting in their first trimester of pregnancy may be more likely to give birth to a child with an oral cleft, including a cleft lip. A growing number of Zofran lawsuits are now moving through U.S. courts that accuse GlaxoSmithKline of failing to warn patients that exposure to the anti-nausea drug in early pregnancy might cause major birth defects.
Expectant mothers who are prescribed Zofran off-label to control nausea and vomiting in their first trimester of pregnancy may be more likely to give birth to a child with an oral cleft, including a cleft lip. A growing number of Zofran lawsuits are now moving through U.S. courts that accuse GlaxoSmithKline of failing to warn patients that exposure to the anti-nausea drug in early pregnancy might cause major birth defects.
A Zofran Lawyer Can Help Your Child
Children born with a cleft lip often require multiple procedures to correct the abnormality, and many experience problems with feeding and speaking. Filing a Zofran cleft lip case could allow your family to obtain compensation for medical bills and other damages related to your child’s birth defect. If you are interested in pursuing a claim against Glaxo, please contact Bernstein Liebhard LLP today, at (888) 994-5118, to discuss your case with one of our Firm’s experienced Zofran lawyers.
Zofran and Pregnancy
The use of Zofran to treat pregnancy-related nausea and vomiting is considered an off-label indication, as the medication has not been cleared for this purpose by the U.S. Food & Drug Administration (FDA). Despite this status, a recent analysis estimated that as many as 1 million expectant mothers annually are prescribed Zofran or a generic equivalent for this purpose.
A growing number of Zofran lawsuits charge that GlaxoSmithKline has engaged in an aggressive campaign to promote the drug as an appropriate treatment for pregnancy-related nausea and vomiting. Among other things, plaintiffs point out that the company paid $3 billion to settle drug marketing charges with the federal government in 2013, including allegations that it had promoted Zofran as a safe and effective treatment for morning sickness.
Zofran and Oral Clefts
Oral clefts form early in pregnancy when the tissue in the mouth does not join together properly. A cleft lip is a physical split or separation of the two sides of the upper lip that looks like a narrow opening or gap in the skin. The gap may extend beyond the base of the nose and into the bones of the upper jaw and/or upper gum.
In most cases, the cause of an oral cleft is unknown. But some recent studies indicate that exposure to Zofran in the first trimester of pregnancy may increase the risk that a baby will be born with a cleft lip or cleft palate.
- 2006: A study called Placental Transfer of Ondansetron During Early Human Pregnancy concludes that the active ingredient in Zofran could cross the human placental barrier during the early months of fetal development, when cleft lips and many other birth defects occur.
- November 2011: Research published in Birth Defects Research linked Zofran to a 2.4-fold increased risk of cleft palate.
- 2013: A study that examined records from 900,000 pregnancies included in a Danish health registry concluded that women who took Zofran during the first trimester were 30% more likely to have a baby with a major birth defect.
Cleft Lip Complications and Treatment
Children who suffer from a cleft lip or palate may experience a number of serious complications:
- Eating problems
- Ear infection/ hearing loss
- Speech delays/problems
- Dental problems
Treatment for oral clefts usually begins when a child is an infant and may continue through early adulthood. Surgical repair of a cleft lip may require two stages, with the first procedure taking place when the child is just 3 months old. Even in severe cases, the long-term prognosis for a child with a cleft lip is very good.
Zofran Lawsuit Updates
Since February 2015, a growing number of Zofran birth defects lawsuits have been filed in courts around the U.S., including a number of Zofran cleft lip and palate cases. In October 2015, all federally-filed Zofran claims were consolidated in a multidistrict litigation that is now underway in the U.S. District Court, District of Massachusetts. More than 100 lawsuits were pending in the proceeding as of November 16, 2015. Read More.
Legal Help for Children with Zofran Cleft Lips
The nationwide law firm of Bernstein Liebhard LLP is now offering free legal reviews to any family who suspect their child developed a cleft lip due to Zofran. To learn more, please call (888) 994-5118.
- AJOG (2014) “Treating morning sickness in the United States—changes in prescribing are needed” http://www.ajog.org/article/S0002-9378%2814%2900853-9/abstract
- Clinical Phamokinetics (2006) “Placental transfer of ondansetron during early human pregnancy” http://www.ncbi.nlm.nih.gov/pubmed/16584287
- Birth Defects Research (2011) “Medications Used to Treat Nausea and Vomiting of Pregnancy and the Risk of Selected Birth Defects” http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3299087/
- TDM Journal Club ( 2013) “Scary Science: Ondansetron Safety in Pregnancy—Two Opposing Results From the Same Danish Registry” http://www.helpher.org/blog/wp-content/uploads/2014/01/2014-Koren-Ondansetron-Safety-TDM.pdf
Get the latest news and litigation updates about this case by following us on Facebook. Click the "Like" button below.
Follow Us


