Subsys Spray
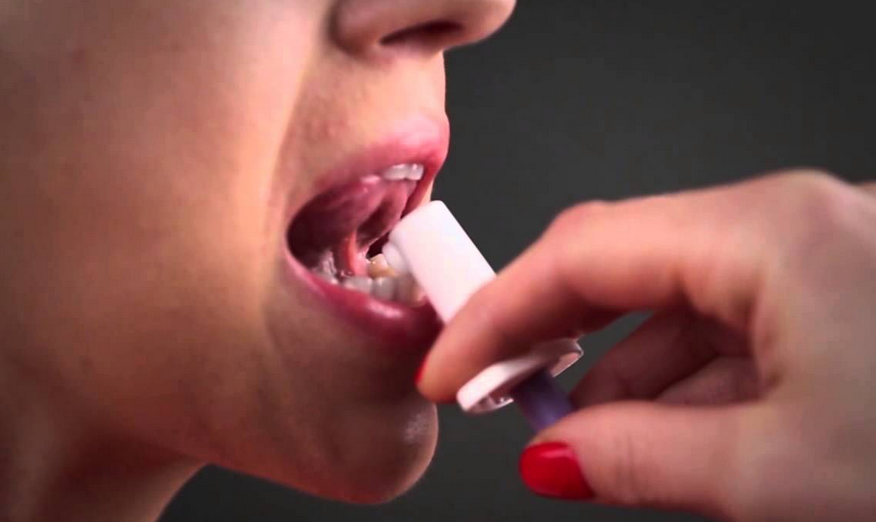
Subsys spray is an opioid painkiller that contains the narcotic fentanyl. The medication is indicated to treat breakthrough pain experienced by adult cancer patients who are already taking opioids and who have developed a tolerance to around-the-clock narcotic pain medicines.
How is Subsys Spray Used?
Manufactured by Insys Therapeutics, Inc., Subsys is a spray version of fentanyl, an opioid narcotic that is 10 times more powerful than morphine. While its approved indications are limited to adult cancer patients who have developed a tolerance to opioid painkillers, the highly addictive drug is frequently prescribed “off label” to non-terminal patients.
The U.S. Food & Drug Administration (FDA) requires that Subsys and the other “TIRF” (Transmucosal Immediate-Release Fentanyl) products be distributed via a restricted program called a Risk Evaluation and Mitigation Strategy, or REMS. According to the agency, a REMS is employed “to manage a known or potential serious risk associated with a medicine and to enable patients to have continued access to such medicines by managing their safe use.”
The REMS for Subsys spray and other TIRF products was developed to mitigate the risk of misuse, abuse, addiction and overdose, as well as serious complications due to medication errors. It is intended to:
- Ensure that TIRF medicines are only prescribed and dispensed to appropriate patients.
- Prevent inappropriate conversion between TIRF medicines.
- Prevent accidental exposure to children and others for whom the medication was not prescribed.
- Educate prescribers, pharmacists, and patients on the potential for misuse, abuse, addiction, and overdose of TIRF medicines
Subsys Spray Black Box Warning
The prescribing information for Subsys spray includes a lengthy Black Box Warning noting the following:
- Subsys spray can be harmful or fatal if taken by children, patients for whom it has not been prescribed, or patients who are not tolerant to narcotic (opioid) pain medicine.
- Subsys spray can cause severe and sometimes fatal breathing problems, especially in those who are not already using other narcotic painkillers or patients who are taking certain other medicine.
- Subsys spray should not be used for short-term pain (including headache, dental pain, or migraine) or for pain that occurs after surgery or injuries.
- Patients should not take more than the recommended dose or use Subsys spray more often than prescribed. Patients should wait at least 4 hours after their last dose before treating a NEW episode of breakthrough pain with the medication.
- Patients should not switch between Subsys spray and other medicines that contain fentanyl without first talking with their doctor. This may cause severe and possibly fatal overdose.
Insys Therapeutics Accused of Illegally Marketing Subsys Spray for Off-Label Use
In December 2016, six former Insys Therapeutics executives, including its CEO, were indicted “on charges that they led a nationwide conspiracy to bribe medical practitioners to unnecessarily prescribe a fentanyl-based pain medication and defraud healthcare insurers.”
The U.S. Department of Justice (DOJ) has alleged that these individuals paid speaking fees to doctors in order to induce them to overprescribe Subsys spray. Ten unnamed medical professionals were listed in the Insys indictment as co-conspirators. Federal prosecutors charge that these individuals wrote prescriptions for non-cancer patients and then conspired with Insys officials in a “reimbursement unit” to write insurance claims that made it appear the patients did have cancer.
In June 2017, a former manager at Insys Therapeutics pled guilty to a single count of wire fraud, admitting that she directed employees at the Insys Reimbursement Center to lie to insurers, defrauding them into paying for Subsys. The manager has agreed to cooperate with the DOJ in its case against the six former Insys Therapeutics executives.
- FDA (2017) https://www.accessdata.fda.gov/drugsatfda_docs/rems/TIRF_2017-04-11_REMS_Full.pdf “TRANSMUCOSAL IMMEDIATE RELEASE FENTANYL (TIRF) RISK EVALUATION AND MITIGATION STRATEGY (REMS)”
- FDA (2012) “Subsys spray: Highlights of Prescribing Information” https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202788s000lbl.pdf
- FDA (2016) “December 8, 2016: Pharmaceutical Executives Charged in Racketeering Scheme https://www.fda.gov/iceci/criminalinvestigations/ucm533555.htm
- com (2017) “Ex-Insys Exec Cops To Opioid Fraud Scheme” https://www.law360.com/articles/936191/ex-insys-exec-cops-to-opioid-fraud-scheme
Get the latest news and litigation updates about this case by following us on Facebook. Click the "Like" button below.
Follow Us


