Endometrial Stromal Sarcoma
The use of a power morcellator during hysterectomy and myomectomy (fibroid removal) may result in the inadvertent spread of undiagnosed endometrial stromal sarcoma and other uterine cancers. U.S. health regulators are now warning against the use of uterine morcellation in the majority of women who require these gynecological procedures.
Legal Help for Power Morcellator Cancer
The nationwide law firm of Bernstein Liebhard LLP is now offering free, no-obligation legal reviews to women who were diagnosed with advanced endometrial stromal sarcoma following uterine surgery with a power morcellator. To learn more about filing an endometrial stromal sarcoma lawsuit, please contact our office at (888) 994-5118.
What is Endometrial Stromal Sarcoma?
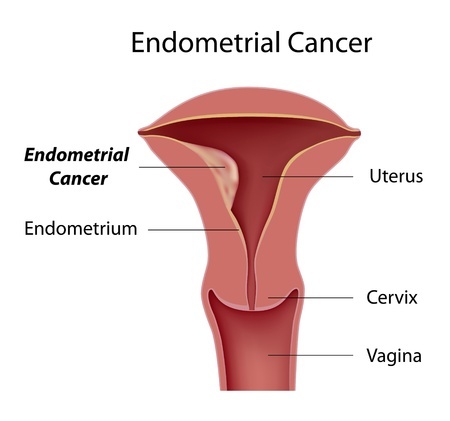
Endometrial stromal sarcoma is one of the rarest forms of uterine sarcoma, and accounts for less than 1% of cancers of the reproductive organs. These malignant tumors occur in the endometrium, the mucous membrane that lines the uterus. This cancer is most often seen in pre-menopausal women in their 40s to 50s. Women in this age group should inform their doctor if they experience heavy periods, bleeding between cycles or after having gone through menopause, or if they are experiencing pain.
Symptoms of Endometrial stromal sarcoma may include:
- A lump or swelling
- Abdominal discomfort or bloating
- Swelling or pain in any area of the body
- Bleeding from the vagina in post-menopausal women
- Change in periods for women who are pre-menopausal
- Abnormal vaginal discharge
- Change in bladder or bowel habits
- Fatigue
- Fever
- Weight loss
- General feeling of ill health
There is no way to diagnose endometrial stromal sarcoma without sampling the uterine tissue. In many cases, the malignancy is initially misdiagnosed as uterine fibroids.
Morcellators and Endometrial Stromal Sarcoma: What’s the Problem?
Power morcellators are often used in minimally-invasive hysterectomies and myomectomies to cut up tissue and fibroids so that it may be removed through a small abdominal incision. If undiagnosed endometrial stromal sarcoma is present at the time of surgery, the morcellator may spread cancer cells beyond the uterus. This can cause the cancer to progress to a more advanced stage and greatly reduce a woman’s chances of long-term survival.
- November 2010: A paper published in Obstetrics and Gynecology details the case of a young woman who was diagnosed with low-grade endometrial stromal sarcoma in her ovary, fallopian tube, and ovarian artery two months after undergoing a morcellator hysterectomy. It is suspected that the power morcellator disseminated undetected cancer cells that were present in her uterus at the time of surgery.
FDA Power Morcellator Warnings
- November 2014: The FDA has issued its second morcellator cancer warning, and specifically cautions against the use of the devices in the majority of women who require hysterectomies and myomectomies. The FDA also orders morcellator manufacturers to add new black box warnings to their product labels.
- April 2014: An FDA communication has discouraged doctors from using power morcellators in hysterectomies and myomectomies. According to the agency, 1 in 350 women undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have an unsuspected uterine sarcoma. The morcellators may spread these malignancies outside of the uterus, worsening the patient’s chance for long-term survival.
Morcellator Lawsuit Developments
- October 2015: Federally-filed product liability claims involving Ethicon morcellators and their alleged propensity to spread undetected uterine cancers have been consolidated in a multidistrict litigation and transferred to one judge in the U.S. District Court, District of Kansas. Read More
- September 2015: The U.S. Judicial Panel on Multidistrict Litigation (JPML) has agreed to consider a motion seeking centralization of all federal morcellator lawsuits. Oral arguments on the matter will be held on October 1st, during the panel’s upcoming Hearing Session in New York City. Read More
- July 2015: Plaintiffs in power morcellator lawsuits are seeking to have all federal cases consolidated in a multidistrict litigation and transferred to a single federal court. However, device manufacturers are opposed to such a move. Read More
- July 2015: Lina Medical ApS settles the nation’s first morcellator lawsuit for an undisclosed amount. The case had been scheduled to go to trial in November 2015. Read More
Filing a Power Morcellator Lawsuit Can Help
Victims of endometrial stromal sarcoma that was spread and upstaged via a power morcellator may be entitled to compensation for medical bills, emotional distress, and other damages. To learn if you qualify to file an endometrial stromal sarcoma lawsuit against the manufacturer of the morcellator used in your surgery, please call (888) 994-5118.
- Obstetrics and Gynecology (November 2010) “Endometrial stromal sarcoma diagnosed after uterine morcellation in laparoscopic supracervical hysterectomy.” http://www.ncbi.nlm.nih.gov/pubmed/20955991
- FDA (November 2014) “FDA warns against using laparoscopic power morcellators to treat uterine fibroids” http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm424435.htm
- FDA (April 2014) “Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication” http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm
Get the latest news and litigation updates about this case by following us on Facebook. Click the "Like" button below.
Follow Us


