Nesina Pancreatic Cancer
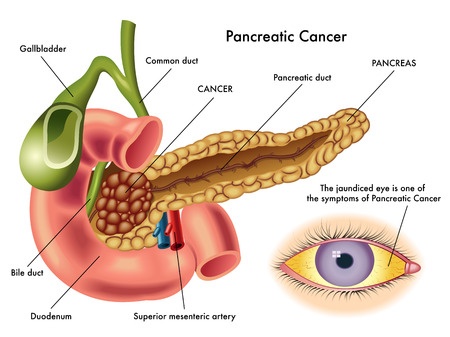
Research has suggested that Nesina and other Type 2 diabetes drugs called incretin mimetics may increase a patient’s risk for pancreatic cancer. Pancreatic cancer is extremely dangerous, with a five- year mortality rate greater than 95%.
Nesina Legal Reviews
Type 2 diabetics who were diagnosed with pancreatic cancer following treatment with Nesina may be entitled to compensation for medical bills, lost wages, pain and suffering and more. To discuss filing a Nesina lawsuit with an attorney at Bernstein Liebhard LLP, please call (888) 994-5118.
Evidence Linking Incretin Mimetics to Pancreatic Cancer
Nesina (alogliptin) works by increasing levels of incretins in the digestive tract. These hormones control blood sugar by stimulating the pancreas to release insulin, especially after a meal. In recent years, a growing body of evidence has suggested that incretin mimetics like Nesina might be associated with a higher risk of pancreatic cancer.
- 2011: The journal Gastroenterology published a study suggesting patients who use incretin mimetics are six times more likely to develop pancreatitis than patients using other diabetes medications. As pancreatitis is a risk factor for pancreatic cancer, the authors of the study concluded that the findings “raised caution about the potential long-term actions of these drugs to promote” the disease.
- 2013: Scientists at Johns Hopkins University reported that patients treated with incretin mimetics were twice as likely to suffer from pancreatitis that required hospitalization.
- 2013: The FDA launches a safety review of incretin mimetics, after a small study reveals inflammation and pre-cancerous cell changes in the pancreatic tissue taken from patients who had used the medications. However, the agency has so far been unable to confirm a link between these drugs and pancreatic cancer.
- 2013: Investigators at the British Medical Journal concluded that pancreatic complications, including cancer, may have been downplayed by the manufacturers of incretin mimetics. Their report also criticized regulators in the U.S. and abroad for not aggressively addressing these concerns.
What is Pancreatic Cancer?
The pancreas is an organ located behind the stomach and in front of the spine, which consists of both exocrine and neuroendocrine cells. Exocrine pancreatic cells produce enzymes that aid in digestion. Neuroendocrine pancreas cells (such as islet cells) make several hormones, including insulin and glucagon, which help control blood sugar levels.
Exocrine cells are where most pancreatic cancers originate. Because these tumors do not secrete hormones or cause much in the way of signs or symptoms, this type of pancreatic cancer is very rarely diagnosed in the early stages. Symptoms that do eventually appear are often vague or non-specific, and may include:
- Pain in the abdomen, stomach, or back
- Indigestion or diarrhea after eating foods high in fat
- Bloating
- Nausea, vomiting
- Loss of appetite
- Unexplained weight-loss
- Tiredness or weakness
- Jaundice (yellowing of the skin and eyes)
- Clay colored stools
- Dark colored urine
Filing a Nesina Lawsuit Can Help
If you or loved one developed pancreatic cancer following treatment with Nesina, the attorneys at Bernstein Liebhard LLP are ready to help. To arrange for your free legal review, please call (888) 994-5118.
- Gastroenterology (2011) Pancreatitis, Pancreatic, and Thyroid Cancer With Glucagon-Like Peptide-1–Based Therapies http://www.gastrojournal.org/article/S0016-5085%2811%2900172-7/fulltext#sec2.1.2
- com (2013) “New generation of diabetes drugs raising more concerns” https://www.minnpost.com/second-opinion/2013/06/new-generation-diabetes-drugs-raising-more-concerns
- FDA (2013) “FDA Drug Safety Communication: FDA investigating reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin mimetic drugs for type 2 diabetes” http://www.fda.gov/Drugs/DrugSafety/ucm343187.htm
- BMJ (2013) “Has pancreatic damage from glucagon suppressing diabetes drugs been underplayed?” http://www.bmj.com/content/346/bmj.f3680
- gov (2015) “Pancreatic Cancer” http://www.cancer.gov/types/pancreatic
Get the latest news and litigation updates about this case by following us on Facebook. Click the "Like" button below.
Follow Us


