MicroPort Profemur Modular-Neck Hip Recall
In August 2015, a MicroPort Profemur Modular-Neck Hip Implant recall was issued for 10,825 Profemur Long Cobalt Chrome 8 Degree Varus/Valgus Modular Neck components (Part 125). The recall was announced after MicroPort Orthopedics, Inc. received 28 reports of the Profemur Modular Necks fracturing. The U.S. Food & Drug Administration (FDA) has declared this action a Class I recall, which indicates that the affected components pose a risk of serious injury or death. Affected patients have been advised to contact their doctors at once if they experience any symptoms of a modular neck fracture.
Microport Profemur Modular-Neck Hip Implant: What’s The Problem?
The component affected by the Profemur Modular Neck recall is used with MicroPort’s Profemur Modular-Neck Hip Implant System. This system consists of interchangeable parts that allow a surgeon to fit the hip implant to each patient’s unique anatomy. The modular neck involved in this recall is a component that fits between the femoral stem and femoral head.
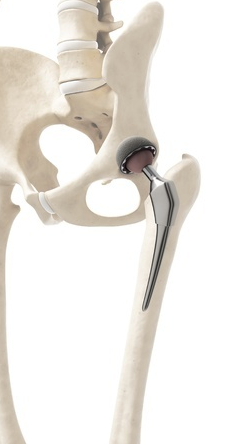 In August 2015, MicroPort announced it was recalling the Profemur Long Cobalt Chrome 8 Degree Varus/Valgus Modular Neck (Part 125) because of an unexpectedly high fracture rate of 0.286%. The FDA has warned that acute fractures of this component may lead to severe health consequences, including:
In August 2015, MicroPort announced it was recalling the Profemur Long Cobalt Chrome 8 Degree Varus/Valgus Modular Neck (Part 125) because of an unexpectedly high fracture rate of 0.286%. The FDA has warned that acute fractures of this component may lead to severe health consequences, including:
- Neurovascular damage
- Hematoma
- Hemorrhage
- Death
According to the FDA, some 10,825 Profemur Modular Necks are being recalled in the U.S. China-based MicroPort has said that 794 units of the affected devices are “currently in the field.”
I’ve Received a Component Included in the Profemur Modular-Neck Recall. What Should I Do?
According to the FDA, patients who suffer a modular-neck fracture may experience:
- Sudden pain
- Instability
- Difficulty walking
- Difficulty performing common tasks
An acute modular neck fracture will require hip revision surgery to remove and replace the neck and stem components. Patients should seek immediate medical treatment if they experience any of the following.
- Sudden onset of severe pain in the hip
- Difficulty or inability walking
- Significant trauma to the hip or leg (e.g. falling)
- Tingling sensation or loss of feeling in the leg
Profemur Modular Neck Patients who have required revision surgery for a recall-related reason may be entitled to compensation for medical bills, lost wages, pain and suffering and more. MicroPort began manufacturing the Profemur Modular-Neck Hip Implant in 2013, after it acquired Wright Medical Group’s OrthoRecon business. Prior to the acquisition, a number of Profemur hip lawsuits had been filed against Wright Medical by individuals who allegedly suffered injuries related to the premature failure of Profemur hip replacements.
If you suffered debilitating complications that may be associated with the MicroPort Profemur Modular-Neck Hip Implant recall, help is available. To learn more about your available legal options, please call (888) 994-5118 to discuss your case with one of our legal experts.
- MicroPort “MINIMALLY-INVASIVE HIP SURGERY (MIS)
PROFEMUR® Modular Neck “ http://www.ortho.microport.com/hipsite/patients/pop_mihsp.asp - MicroPort (August 2015) “Voluntary Announcement – Voluntary Recall of Modulra Neck Part” http://www.microport.com.cn/en/attach/investor_pic/investors/announcements/15072708_PAe10Aug2255.pdf
- FDA (August 2015) “MicroPort Orthopedics Inc., PROFEMUR Neck Varus/Valgus CoCr 8 Degree, Part number PHAC 1254” http://www.fda.gov/MedicalDevices/Safety/ListofRecalls/ucm465501.htm
- Wright Medical Group (June 2013) “Wright Medical Group, Inc. and MicroPort Scientific Corporation Enter Into Definitive Agreement Under Which MicroPort Will Acquire Wright’s OrthoRecon Business” http://www.businesswire.com/news/home/20130619006505/en/Wright-Medical-Group-MicroPort-Scientific-Corporation-Enter#.Vhacuyt1yUQ
- Wright Medical Group (February 2015) “Form 10-K” http://www.sec.gov/Archives/edgar/data/1137861/000113786115000010/wmgi-12312014x10k.htm
Get the latest news and litigation updates about this case by following us on Facebook. Click the "Like" button below.
Follow Us


