Kombiglyze XR Lawsuit
A recent study has suggested that Type 2 diabetics using Kombiglyze XR are more likely to be hospitalized for a heart failure diagnosis compared to those taking other diabetes medications. Filing a Kombiglyze lawsuit could enable victims of this potential side effect to obtain compensation for their pain and suffering.
Filing a Kombiglyze Lawsuit
The nationwide law firm of Bernstein Liebhard LLP has helped hundreds of people who suffered physical, financial and emotional losses due to serious drug side effects. If you would like to learn more about filing a Kombiglyze heart failure lawsuit, please call (888) 994-5118.
What is Kombiglyze?
Kombiglyze XR belongs to a class of Type 2 diabetes medications called DPP4-ihibitors. It combines two drugs, saxagliptin (the active ingredient in Onglyza) and metformin. Kombiglyze XR is marketed by AstraZeneca, and was approved by the U.S. Food & Drug Administration (FDA) in 2010.
Breaking Kombiglyze XR News: FDA Warns of Heart Failure Risk, Adds New Label Warnings
The FDA announced on April 5, 2016 that new Warnings and Precautions regarding a potential risk of heart failure would be added to the label of Kombiglyze XR. The announcement followed the review of a clinical trial in which 3.5 percent of patients receiving saxagliptin were hospitalized for heart failure, compared with 2.8 percent of patients receiving a placebo. Risk factors included heart and kidney disease. Read More
The FDA has cautioned that patients should not stop taking their Type 2 diabete medicine without first talking to their health care professionals. Kombiglyze XR patients should contact their doctor if they experience any of the following heart failure symptoms:
- Unusual shortness of breath during daily activities
- Difficultly breathing when lying down
- Tiredness, weakness, or fatigue
- Weight gain with swelling in the ankles, feet, legs, or stom
Kombiglyze XR: What’s the Problem?
Since 2014, concerns surrounding the safety of saxagliptin have grown, prompting the FDA to launch a safety review of both Kombiglyze XR and Onglyza.
- October 2013: Findings from the SAVOR TIMI trial are published in the New England Journal of Medicine, and suggest that patients using saxagliptin may be 27% more likely to be hospitalized for heart failure. The study followed more than 16,000 Type 2 diabetics treated with either saxagliptin or a placebo for a median of 2.1 year.
- February 2014: The FDA launches a safety review of both Kombiglyze XR and Onglyza, based on the heart failure results of the SAVOR TMI study.
- April 2015: Fourteen of 15 members of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee recommend that new information regarding a potential association with heart failure be included on the labels for Kombiglyze XR and Onglyza. While the FDA is not required to follow the recommendations of its advisory panels, it usually does so.
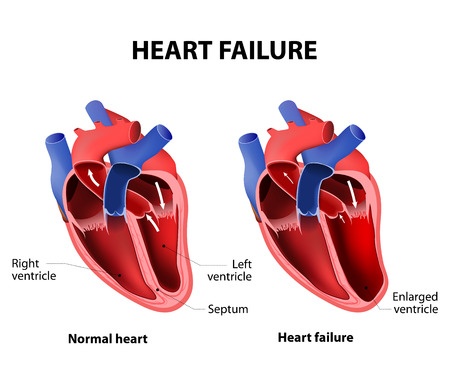
What is Heart Failure?
Heart failure occurs when the heart is unable to effectively pump oxygenated blood to other parts of the body. According to the American Heart Association, the warning signs and symptoms of heart failure include:
- Shortness of breath (dyspnea)
- Persistent coughing or wheezing
- Edema (buildup of excess fluid in body tissues, may present with swollen ankles or feet)
- Tiredness, fatigue
- Lack of appetite, nausea
- Cognitive impairment
- Rapid heart beat, palpitations
While heart failure is a chronic, progressive condition, many people can and do learn to manage its symptoms and live full and enjoyable lives.
Litigation Involving Kombiglyze XR and Onglyza
- October 2015: An Onglyza lawsuit filed in in Illinois’ Cook County Circuit Court claims that saxagliptin caused the death of a woman from heart failure-related complications. The wrongful death claim seeks compensation on behalf of the woman’s daughter. Read More
Legal Reviews for Those Harmed by Kombiglyze
If you are interested in pursuing a Kombiglyze lawsuit for heart failure, the attorneys at Bernstein Liebhard LLP can evaluate your claim at no cost or obligation to you. To get in touch with our Firm, please call (888) 994-5118.
- FDA ( 2013) “Kombiglyze XR Medication Guide” http://www.fda.gov/downloads/Drugs/DrugSafety/UCM280360.pdf
- NEJM (2015) “Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus” http://www.nejm.org/doi/full/10.1056/NEJMoa1307684
- FDA (2014) “FDA Drug Safety Communication: FDA to review heart failure risk with diabetes drug saxagliptin (marketed as Onglyza and Kombiglyze XR)” http://www.fda.gov/Drugs/DrugSafety/ucm385287.htm
- American Heart Association (2015) “Heart Failure” http://www.heart.org/HEARTORG/Conditions/HeartFailure/Heart-Failure_UCM_002019_SubHomePage.jsp
Get the latest news and litigation updates about this case by following us on Facebook. Click the "Like" button below.
Follow Us


