Duodenoscopes
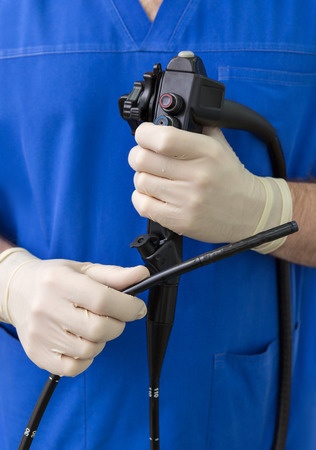
Duodenoscopes are medical instruments used to perform a diagnostic procedure called endoscopic retrograde cholangio-pancreatography (ERCP). While complications from ERCP are rare, a number of hospitals have recently reported dangerous multidrug-resistant bacterial infections that likely originated with contaminated duodenoscopes.
What is a Duodenoscope?
Every year, some half a million U.S. patients undergo duodenoscope -assisted ERCP to examine and detect diseases of the liver, bile ducts and pancreas. These long, flexible, instruments are about the diameter of a pen, and are equipped with a tiny light and camera at one end. Three device manufacturers currently market duodenoscopes in the U.S.:
- Olympus America Inc.
- Pentax
- Fujifilm
What is ECRP?
During ERCP, a duodenoscope is inserted into a patient’s mouth and guided through the esophagus, stomach and duodenum. It then transmits digital video images to a TV screen. Contras dye is injected through an open channel of the scope and X-rays are taken of the bile ducts and the pancreatic duct. The duodenoscope’s open channel also allows instruments to be passed through in order to perform biopsies, relieve obstructions of the bile ducts or pancreas, and to create incisions via electrocautery.
For best results, ERCP should be performed on an empty stomach. Prior to the procedure, the patient will be administered IV medications to cause relaxation and sleepiness, and possibly a local anesthetic to decrease the gag reflex. During ERCP, the patient will be in a semi-conscious state, and will still be able to follow instructions to change body position on the X-ray table. The procedure can last anywhere from fifteen minutes to one hour.
Duodenoscope Complications
While ECRP is generally a safe procedure with a success rate of 75% to 90%, roughly 1 in 5 patients will experience complications. Some of the more serious adverse events include:
- Pancreatitis resulting from irritation of the pancreas
- Intestinal perforations
- Drug reaction
- Bleeding
- Depressed breathing
- Irregular heart beat or heart attack.
Duodenoscope Infections
Recently, bacterial infections have come to be recognized as serious and potentially deadly duodenoscope complications.
- February 2015: The S. Food & Drug Administration (FDA) warned that the complex design of duodenoscopes can impede efforts to clean, disinfect or sterilize reusable devices. From January 2013 through December 2014, the agency received 75 reports of possible microbial transmission from reprocessed duodenoscopes involving 135 patients. However, it is possible that additional cases occurred but were not reported to the FDA.
- May 2015: A panel of FDA medical advisors determined that ERCP duodenoscopes “do not provide a reasonable assurance of safety and effectiveness,” but stopped short of recommending that they be recalled. Read More
- August 2015: the FDA issued a second communication with expanded duodenoscope cleaning procedures. T he agency also sent warning letters to Olympus, Pentax and Fujifilm, accusing the duodenoscope manufacturers of failing to report problems with their instruments. Read More
- com (September 2014) “ERCP
(Endoscopic Retrograde Cholangio-Pancreatography)” http://www.medicinenet.com/ercp/article.htm - FDA (February 2015) “Design of Endoscopic Retrograde Cholangiopancreatography (ERCP) Duodenoscopes May Impede Effective Cleaning: FDA Safety Communication” http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm434871.htm
- LA Times (May 2015) “Federal panel calls medical scopes unsafe” http://www.latimes.com/business/la-fi-scope-infections-20150516-story.html
- FDA (August 2015) “Supplemental Measures to Enhance Duodenoscope Reprocessing: FDA Safety Communication” http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm454766.htm
- Bloomberg (August 2015) “FDA Issues Warning to Scope Makers Over Spread of Deadly Superbugs” http://www.bloomberg.com/news/articles/2015-08-17/fda-issues-warning-to-scope-makers-over-spread-of-deadly-superbugs
Get the latest news and litigation updates about this case by following us on Facebook. Click the "Like" button below.
Follow Us


